RNMC

Reaction Network Monte Carlo

Reaction Network Monte Carlo
Two examples of applications of LGMC, CO Oxidation on Cu with a static lattice, and a simple reaction network for the formation and evolution of the solid electrolyte interphase (SEI) in a lithium-ion battery, are provided.
This example simulates electrocatalytic CO oxidation on Cu. We employ a static lattice with 50 sites in each of the x and y dimensions and 2 sites in the z dimension to represent a Cu surface as the catalysis.
All initial sites are empty. The initial state of the solution in contact with this surface consists of 2,500 CO molecules and 15,000 HO molecules. The reduction and oxidation rates use an electron free energy of -0.5 eV. The system is modeled at 300K. Butler-Volmer electron transfer theory is used through the simulation.
The allowed reactions and associated rates are as follows:
| Reaction | Prefactor | Rate (s-1) |
|---|---|---|
| COsoln + * → CO* | 1 | 105 |
| * + H2O(soln) → H2O* | 1 | 102 |
| H2O* → * + H2O(soln) | 1 | 102 |
| H2O → OH + e- | 0.02 | 0 |
| OH* + e- → H2O* | 104 | 0 |
| CO* + OH* → CO2* + e- | 0.8432 | 0 |
| CO2* → * + CO2(soln) | 1 | 104 |
| CO* + * → * + CO* | 1 | 1 |
| OH* + * → * + OH* | 1 | 1 |
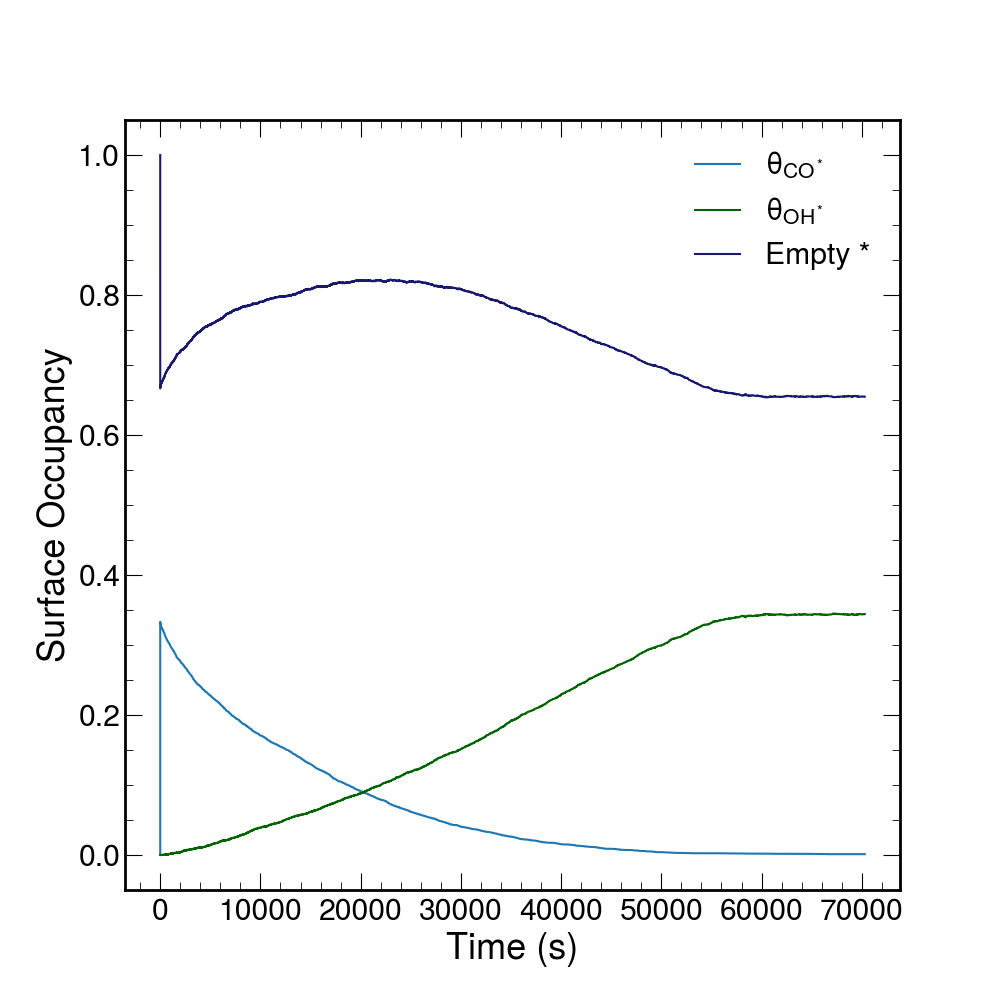
From this initial state, 200,000 steps of our kMC are run. As the simulation proceeds, CO and H2O rapidly adsorbs onto the lattice. The H2O on the lattice then oxidizes to form OH which reacts with the CO on the lattice to form CO2. This CO2 lattice product then desorbs into the solution. The results of the simulation are shown as the occupancy of the lattice sites.
This example simulates a simplified formation and evolution of the solid electrolyte interphase in a lithium-ion battery. We employ a dynamic lattice with 100 sites in both the in each of the x and y dimensions and 2 sites in the z dimension to represent the interphase between the elctrode and electrolyte in a lithium-ion battery.
All initial sites are empty. The initial state of the solution in contact with this surface consists of 10000 LiEC+ molecules.The reduction rates use an electron free energy of -2.1 eV. The system is modeled at 300K. Marcus electron transfer theory is used through the simulation.
Because there are 99 total reactions allowed to occur in the simulation, a brief overview is provided instead of a table. To see each reaction see the Python and .sqlite files in examples directory .
EC0, EC-, LiEC+, LiEC0, LiEC_RO0, LiEC_RO-, LiCO3-, Li2CO30, LEDC0, LEDC-, LEDC_minus_Li-, LEDC_plus_Li+, LEDC_plus_Li0, C2H40